Alpharetta-Based Annoviant is a Medical Device Startup That Truly Grows With Patients
ALPHARETTA, Ga. — A local startup CEO left the corporate world to pursue a solution for one of the world’s most pervasive health problems.
“We can save lives,” Dr. Ajay Houde, CEO and co-founder of Annoviant, said.

In 2018, Houde left Halyard Health, formerly a part of Kimberly-Clark, a Fortune 100 company with operations in Alpharetta, to pursue a breakthrough technology to help patients with damaged or defective hearts. He and co-founder Dr. Naren Vyavahare, whom Houde described as the “brain behind the technology,” created the Alpharetta-based startup Annoviant and an innovative process, dubbed TxGuard.
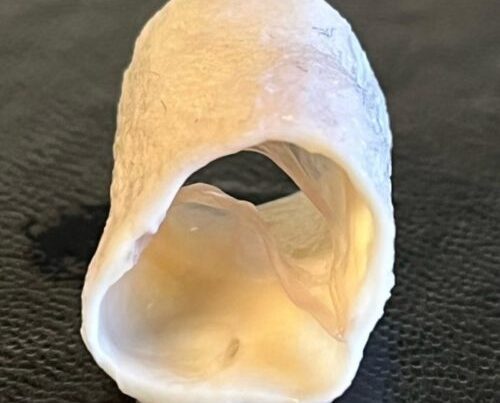
TxGuard technology removes animal cells from an animal’s blood vessel through a proprietary process and implants it into a person’s heart, allowing it to serve as a kind of scaffold for human cells to grow around. The technology’s ability to regenerate and grow with patients’ bodies may make it especially helpful to children born with heart defects.
Children born with heart defects have few options for replacing heart valves, Vyavahare said.
Annoviant has so far secured four grants from the National Institute of Health and about $7.5 million in funding.
“We can give a better option, minimize the number of repeat procedures and save the time in the hospital for people,” he said.
TxGuard, Houde said, has the potential to make an impact on a huge health problem.
Almost 400,000 people undergo heart bypass surgery in the U.S. each year, according to WebMD. Traditionally, the surgery involves taking a blood vessel from another part of the body to go around a blocked cardiac artery or defective parts of the heart.
Since the 1970s, the methods have largely remained the same. Although the surgery saves lives and has a high survival rate, the procedure often requires patients to take anticoagulant drugs and undergo repeat surgeries as the replaced tissues harden.
Developing the technology behind TxGuard has been difficult at times, Houde said, but his business has received support from nonprofit startup incubator Tech Alpharetta, as well as other organizations. That support, Houde said, has proved invaluable.
“This has been, for me, a lifesaver,” he said.
With dozens of startups partnered with Tech Alpharetta, the nonprofit has served as a place where Houde can seek mentorship, discuss new ideas and find help in solving complex problems.
The support from Tech Alpharetta has been a blessing, says Houde, but also has offered him motivation in personal tragedy.
A few months ago, one of Houde’s close relatives was diagnosed with coronary heart disease.
“So many people are struggling,” Houde said, adding, “There are 20 million people today who are struggling with coronary heart disease.”
Other people close to Houde also have experienced serious health issues. In 2017, his mother died because of a lack of proper diagnosis. In 2002, his wife was rushed to the hospital to receive a stent to treat a renal artery defect. And his sister has struggled with breast cancer.
The health difficulties experienced by Houde’s loved ones were one major reason he decided to leave his position in the corporate world.
“Health care is actually very near and dear to me because I have lost many of my relatives, some close friends,” he said. “Some of my family members also were impacted by a lack of having the proper diagnostic or proper treatment.”
Annoviant’s TxGuard aims to provide heart disease patients better options.
During coronary heart surgery, physicians normally remove a vein from a patient’s leg to bypass one in their heart. But for some people, like the elderly or those who have diabetes, that may not be a viable option.
Although the TxGuard technology has not yet been tested in humans, Houde said that could be coming relatively soon. Annoviant has been working with the FDA throughout its development process. He said getting FDA approval could nevertheless take a number of years.
The first TxGuard devices would likely be implanted on pediatric patients who have few options, he said.
“Saving lives and making people healthy is a noble cause,” Vyavahare said. “And it gives immense inner joy if you even help one patient.”