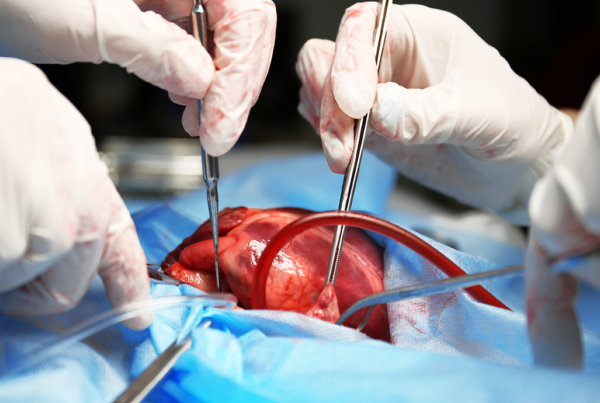
Annoviant is Developing the Next Generation of Biomaterials to Fabricate Cardiovascular Devices for the Pediatric Population
Congenital Heart Disease (CHD) affects approximately 40,000 newborns each year in the U.S. and 1.3M children globally. Valve and conduit replacements are life-saving for children with absent pulmonary valves, aortic stenosis (Konno procedure, Ross, procedure), double outlet right ventricle (Rastelli operation), extracardiac conduit (Fontan operation), and pulmonary valve replacement (Tetralogy of Fallot). According to the Centers for Disease Control and Prevention (CDC), there are about 2.9 million CHD patients in the U.S. The primary issues currently observed with commercial devices are calcification, thrombosis, and infection. The current devices need to be replaced multiple times as children grow with an estimated total cost to the U.S. healthcare industry for treatment of CHD patients of $5.61 billion.
Annoviant LLC, formerly TGen Tech LLC, is a medical device company founded in 2018 and collaborating with a broad ecosystem of research institutions and industry partners across the country. Fueled by personal experiences of its co-founders, the company is driven by a passion to make a positive difference in the lives and treatment of congenital heart disease (CHD) patients. The company has developed a platform decellularized tissue based TxGuard™ technology that make such devices resistant to calcification, thrombosis, and infection. The ability of these tissues to remodel through infiltration with the patient’s own cells without compromising function also is a distinguishing benefit. The company is developing cardiovascular devices for structural heart treatments. The new TxGuard™ portfolio of devices can address the clinical challenges and significantly reduce healthcare costs by eliminating repeat surgeries.
The Pennsylvania Pediatric Device Consortium (PPDC) supported Annoviant’s earlier work and connected company to the FDA for initial discussions. This support was essential for the company to obtain substantial NIH funding later in its development. Annoviant has received two NIH Phase I STTR and one Phase II SBIR grants to commercialize the TxGuard™ pulmonary valve conduit and bring it into clinical use.
There are several next steps that the team at Annoviant is currently working on including:
- Translating the technology to create commercial scale GMP production and GLP testing.
- Finding the appropriate regulatory pathways for the technology, recognizing that Annoviant’s first device has received HUD designation from FDA.
- Continuing to develop and test products with a focus on the sector of pediatric disease, recognizing that other larger companies may be more reluctant to enter the market due to large upfront R&D expenses and risks.
Annoviant is Developing the Next Generation of Biomaterials to Fabricate Cardiovascular Devices for the Pediatric Population